Nirmatrelvir/ritonavir (NMV/r) is the first FDA approved oral antiviral indicated for the treatment of mild-to-moderate COVID-19 in individuals at a high risk of severe illness. Although NMV/r was approved by FDA in May 2023, the commercialization and price setting of the treatment for health plans was delayed until the end of 2023 as drug access was maintained through a federally purchased supply until depletion. Now that commercial plans are responsible for the acquisition cost of NMV/r, a budget impact analysis is of key interest to US payers.
We developed a budget impact model to assess the impact of NMV/r on health care costs in a hypothetical 1-million-member commercial health insurance plan over a 1-year period.
Clinical and cost inputs were derived from published literature with a focus on studies in the recent COVID-19 era that included vaccinated population and predominance of the Omicron variant. In the base-case analysis, it was assumed the only effect of NMV/r was a reduction in incidence (not severity) of hospitalization or death; its potential effect on post-COVID conditions was assessed in a scenario analysis. Outcomes included the number of hospitalizations, total cost, per patient per year (PPPY) costs, and per member per month (PMPM) costs. Sensitivity and scenario analyses were conducted to assess uncertainty around key model inputs.
An estimated 29,999 adults were eligible and sought treatment with oral antiviral for COVID-19 over 1 year. The availability of NMV/r was estimated to reduce the number of hospitalizations by 647 with a total budget impact of $2,733,745, $91 PPPY, and $0.23 PMPM.
NMV/r was cost saving when including post-COVID conditions with a -$1,510,780 total budget impact, a PPPY cost of -$50, and a PMPM cost of -$0.13.
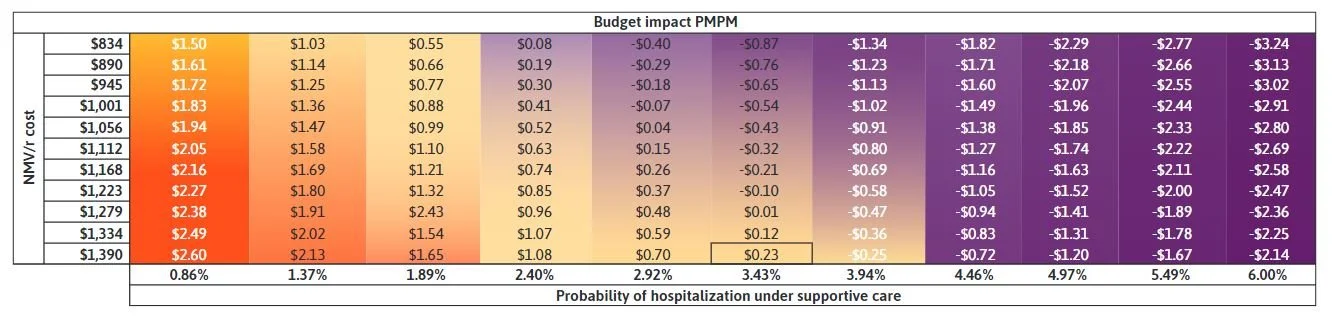
Read the full article here: https://www.jmcp.org/doi/10.18553/jmcp.2023.29.12.1290