Colorectal cancer (CRC) is a leading global cause of cancer-related deaths. Recent clinical trials have suggested a potential role for the human epidermal growth factor receptor 2 (HER2) as a significant biomarker in CRC. However, routine testing for HER2 in CRC is not yet standard practice, resulting in an uncertain understanding of the prevalence of HER2 positivity (HER2+) in CRC patients.
To address this knowledge gap, we conducted a systematic literature review and meta-analysis, encompassing 224 studies with relevant information about HER2 in CRC. Among these studies, 52 employed FDA-approved assays and were chosen for further analysis.
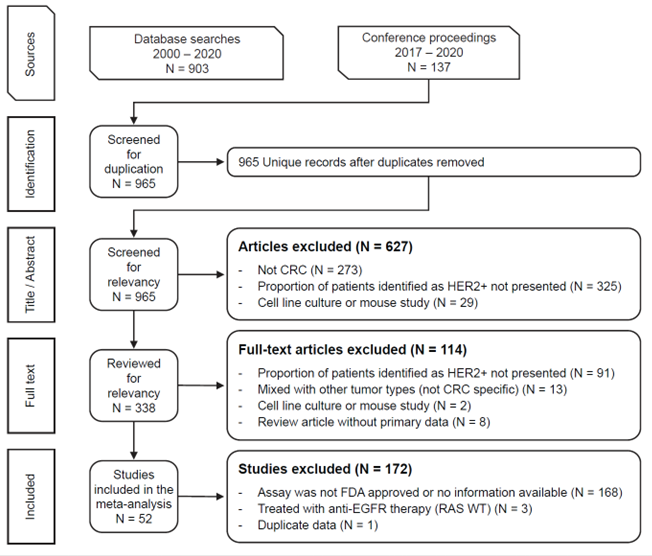
Figure 1. PRISMA Diagram
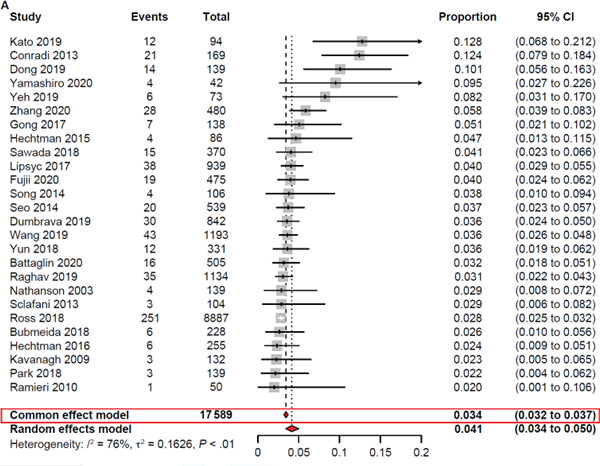
The results of this comprehensive analysis indicated an estimated HER2+ rate of 4.1% in CRC patients (Figure 2), with a higher prevalence of 6.1% in those with RAS wild-type (WT) CRC. In contrast, HER2+ was found in only 1.1% of CRC patients with RAS mutant tumors.
Figure 2. Meta-analysis of HER2+ in the overall CRC population
Moreover, despite limited available clinical data, the meta-analysis confirmed a significant enrichment of HER2+ CRC in patients with microsatellite-stable tumors and left-sided CRC. The findings suggest that the clinical efficacy of HER2-targeted therapies in HER2+ CRC is becoming increasingly recognized. As a result, we recommend the incorporation of HER2 testing as part of the standard care for CRC patients, particularly those with RAS-WT and left-sided CRC, in order to identify candidates who may benefit from HER2-targeted therapies.